Quantum Mechanics: Schrödinger Equation
Quantum Mechanics: Schrödinger Equation
We had learned a lot about wave-particle duality and about
these subatomic particles.
We need a theory to describe their behavior and classical mechanics had some flaws. We need quantum mechanics.
Topics
- The Schrödinger Equation
- Time independent Schrödinger Equation (TISE)
- Time dependent Schrödinger Equation (TDSE)
The Schrödinger Equation is an equation of the motion of particles (like electrons) that account for their wave-like properties.
The Schrödinger Equation is to the quantum mechanics like Newton's equation of motion are to classical mechanics.
Mathematical representation of The Schrödinger Equation is
This is the simplest form of the equation that you will probably ever see.
Ψ = Wavefunction, to know more about wavefunction visit my blog on the wavefunction @ https://allnotes2020.blogspot.com/2020/06/importantterms.html
E = Binding energy, that's the energy of the binding an electron to the nucleus.
Schrödinger Equation for Hydrogen
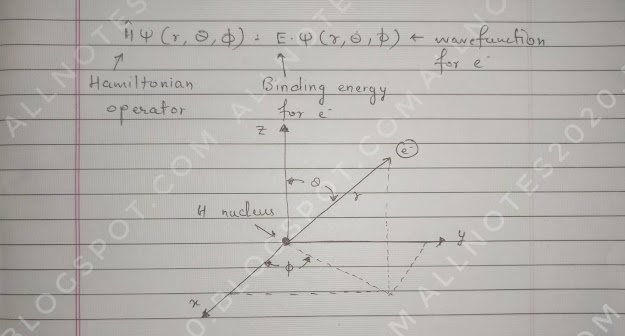 |
| Click on the image for a better view. |
You must be thinking about really what this equation is doing and where it came from, how this solving help us, what are we actually learning?
One thing we learn from this is that we find E(binding energy) and that is really important, the binding energy of the nucleus and electron.
We know that electron and proton are of opposites charges and why don't they crash into each other we want to know how they are bonded to each other, what's the real energy of that association.
We know from the photoelectric effect that it's not easy to get an electron to eject from a metal surface, and that's what the binding energy is. So that comes out of the Schrödinger equation and the second thing is after solving it will tell us about the wave function. So it predicts E, Ψ, and stable H atom in agreement with our observations.
 |
| Click on the image for a better view. |
BINDING ENERGIES ARE QUANTIZED!
The principal quantum number comes out of the Schrödinger equation.
TIME DEPENDENT SCHRÖDINGER EQUATION
TIME INDEPENDENT SCHRÖDINGER EQUATION
Follow this blog page to get updates on the upcoming posts. Stay Tuned.
Thank you.
~Amol Pandit.








Very nice notes sir !! The way you derive is easiest one
ReplyDelete